Abstract
Background:
Hemophilia A results from a mutation in the gene for coagulation factor VII and serious disease can cause bleeding in soft tissues and joints. These patients rely on exogenous factor VIII. However, frequent infusions are needed due to the short half-life of factor VIII which can lead to non-compliance to therapy and poor quality of life. Gene therapy aims to safely impart the expression of stable factor VIII. In this review, we will assess the efficacy and safety of gene therapy in hemophilia A.
Methods:
We searched PubMed, Web of Science, and clinicaltrials.gov with mesh terms, "hemophilia A" and "genetic therapy" from the inception of data till May 30, 2022. Out of 6,699 articles, 4 clinical trials (N=173) were included based on inclusion criteria measuring the efficacy and safety of gene therapy in hemophilia A. Case reports, review articles, systematic reviews, meta-analyses, pre-clinical studies, and clinical trials with irrelevant treatment or population were excluded.
Results:
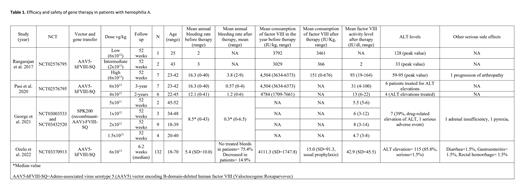
In 4 clinical trials, 173 adult patients (<18 years old) were treated with vector-based gene therapy. Adeno-associated virus serotype 5 (AAV5) vector was used in 155 patients while SPK-8011 was used in 18 patients. 146 patients were treated at a high dose of 6x1013 vg/kg. All patients had a factor VIII activity of <1 IU/dl before therapy. In 2 clinical trials (N=139), mean factor VIII activity significantly increased to 45.42 IU/dl on treatment with 6x1013 vg/kg of AAV5-hFVIII-SQ. However, the activity level was ≤2 IU/dl after treatment at lower doses. On three years follow-up by Pasi et al. (N=7), the mean factor VIII activity level was 31 IU/dl. The mean annual bleeding rate decreased from 5.95 (N=139) to 0.95 after therapy. Mean consumption of factor VIII decreased from 4130.8 (N=139) to 21.84 IU/kg after treatment with 6x1013 vg/kg of AAV5-hFVIII-SQ. In one clinical trial by George et al., 18 patients were treated with SPK-8011-FVIII-SQ and the median annual bleeding rate decreased from 8.5 to 0.3. Mean factor VIII activity was 8 at a dose of 2x1012. ALT elevations, adrenal insufficiency, pyrexia, diarrhea, rectal hemorrhage, and progression of arthropathy were among the serious side effects reported in patients with gene therapy. Table 1.
Conclusion:
Gene therapy was well tolerated by most of the patients and ALT elevation was the most common adverse effect reported. AAV5-hFVIII-SQ and SPK-8011-FVIII-SQ significantly improved the endogenous production of factor VIII, bleeding rate, and factor VIII concentrate consumption, especially at doses of 6x1013 and 2x1012 vg/kg, respectively. AAV5-hFVIII-SQ was safe and effective in patients with hemophilia A on long-term follow-up. More randomized double-blind clinical trials are needed to confirm these results.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.